"This is the clearest three-dimensional representation of the macaque monkey brain structure to date and marks an important step toward ultimately understanding the human brain." Professor Guoqiang Bi from the Hefei National Research Center for Microscale Material Science at the University of Science and Technology of China and the Brain Cognitive Science and Brain Disease Institute at the Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, told DeepTech.

GIF | VISoR high-speed 3D imaging technology shows neural fibers in the macaque monkey brain (Source: Nature Biotechnology)
Neural fibers are the carriers of information transmission in all consciousness and thought activities within the brain. Each neuron in the brain has a unique structure that can grow long fiber branches projecting into different regions of the brain, thus forming complex network connections and exchanging information.
Therefore, resolving such ultra-fine details within the brain is no easy task, which requires achieving subcellular resolution through three-dimensional imaging.
The characteristic of neural fibers is being "thin and long," with diameters typically less than 1 micrometer—about 1/100th the thickness of a human hair strand—and lengths reaching up to tens of centimeters, hundreds of thousands of times their diameter. Additionally, sometimes these fibers extend through numerous branches covering nearly half the brain, making localized imaging using high-resolution methods like electron microscopy insufficient to capture even the complete view of a single neuron.
Thus, there is a need for a three-dimensional high-definition "map" capable of simultaneously meeting both requirements of high precision and wide range to achieve long-distance tracing of neural fibers.
Recently, collaborative teams including the University of Science and Technology of China, the Brain Cognitive Science and Brain Disease Institute at the Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, and the Artificial Intelligence Research Institute at the Hefei Comprehensive National Science Center have independently developed a high-throughput, high-precision 3D fluorescence imaging VISoR technology and a SMART workflow for mapping primate brains, enabling micron-level 3D resolution analysis of the macaque monkey brain.
VISoR imaging systems can complete image acquisition of a macaque whole-brain sample at a 1×1×2.5 micrometer 3D resolution within 100 hours. The original data volume from the two macaque brain images used in this study exceeded 1 PB (quadrillion bytes), equivalent to the storage capacity of 113 ten-terabyte hard drives. This represents the highest-resolution brain atlas of primates achieved anywhere in the world to date.

Image | Related paper (Source: Nature Biotechnology)
On July 26, related research was published in Nature Biotechnology under the title “High-throughput mapping of a whole rhesus monkey brain at micrometer resolution.” Professors Guoqiang Bi and Beiming Liu served as corresponding authors, while Associate Researchers Fang Xu from the Shenzhen branch of the University of Chinese Academy of Sciences and Shenzhen Institutes of Advanced Technology, and graduate students Yan Shen, Lufeng Ding, Chaoyu Yang from USTC and guest researchers at the Brain Institute participated as first authors.

Image | Neurons in the macaque monkey brain (Source: Interviewee)
So, what insights can we gain from these images? What will they tell us? A user on Twitter commented: It may reveal thousands of things, just like an Earth "map"—it depends on what questions you use this tool to ask.
Fang Xu told DeepTech that macaques are the most suitable non-human primate model animals for studying human intelligence and the mechanisms behind human brain diseases. This technology opens a door for mesoscopic connectome studies of primate brains. With such a detailed 3D "map," it becomes possible to truly understand and simulate the brain’s working mechanisms, paving the way for designing future brain-inspired intelligent systems.
Independent development of high-speed 3D imaging technology, acquiring 3D high-definition imaging of the macaque monkey brain for the first time

Typically, fluorescence micro-optical sectioning tomography (fMOST) and serial two-photon tomography (STPT) take days to complete imaging of a mouse brain, whereas using the team’s self-developed VISoR high-speed 3D imaging technology takes only 0.5–2 hours, increasing speed by dozens of times.
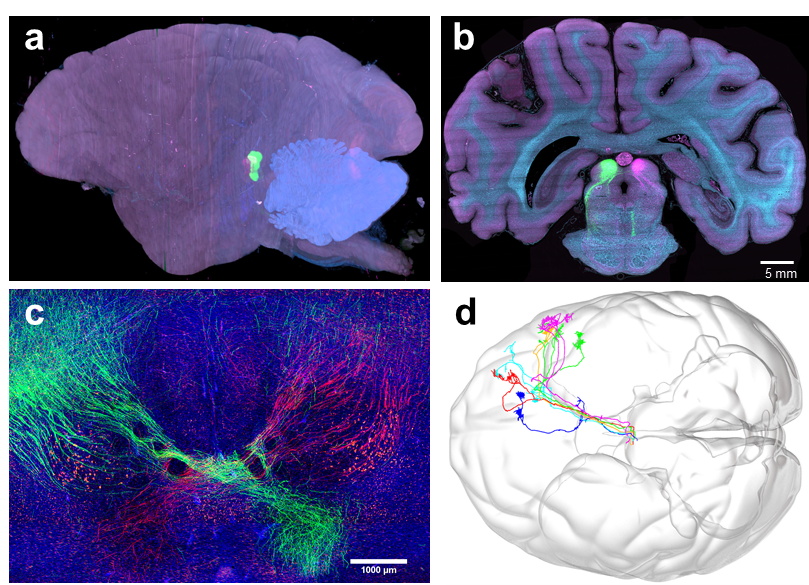
Image | High-resolution 3D reconstruction of the macaque brain (a), cross-section (b), display of internal neural fibers (c), and whole-brain tracing and visualization of some neural fibers (d) (Source: Nature Biotechnology)
The team began developing the VISoR high-speed 3D imaging technology in 2015 under support from the Chinese Academy of Sciences’ Strategic Priority Research Program and the National Natural Science Foundation of China, aiming to meet widespread needs in brain science research, obtaining 3D structural images of experimental animal brains, and mapping neural connectivity across different scales.
Fang Xu told DeepTech that the principle of this technology is similar to taking panoramic photos with a smartphone. To achieve mesoscale 3D brain imaging, it is necessary to switch between different fields of view, photographing each small area of the brain sample separately, and finally stitching together millions of small images into a complete 3D image. Therefore, imaging speed is crucial.
The fundamental reason traditional technologies are slow is because the sample remains stationary during imaging. When transitioning from one field of view to another, the sample must accelerate, move a certain distance, and then decelerate back to stillness.
Video | Neural fiber structure in the macaque brain reconstructed using the SMART workflow (Source: Nature Biotechnology)
In these steps, the camera exposure time while the sample is stationary is very short, but the time spent switching between views far exceeds the exposure time. In other words, the camera is idle most of the time, and its imaging speed is wasted in "waiting."
If light-sheet illumination imaging is combined with transparent brain techniques, although faster, the resolution is far from sufficient. Given this, Dr. Qingyuan Zhu, a senior engineer in the team, proposed a new imaging mode: Why not try continuous shooting while the sample is moving at a constant speed? This allows maximum time efficiency and achieves the camera's ultimate imaging speed.
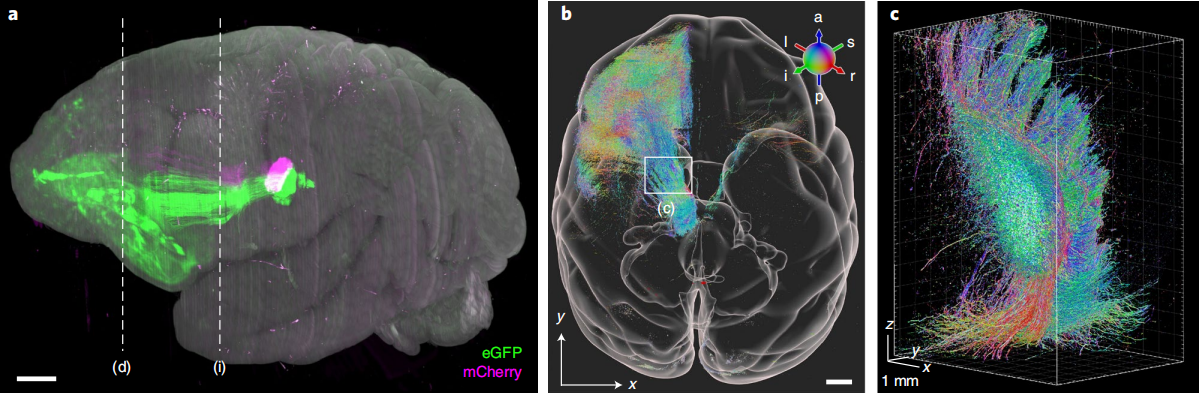
Image | Mesoscopic projections of thalamic nuclei in the macaque (Source: Nature Biotechnology)
However, another issue arises: imaging while moving can easily cause image blur due to instability. How can this be resolved?
As explained, another key aspect of the technology was conceived by Professor Guoqiang Bi—the use of scanned beam illumination. “We strictly synchronize the scanning beam with camera exposure and readout. Each point in the sample is illuminated only once, almost completely avoiding imaging blur caused by sample movement instability. Moreover, this imaging method efficiently uses light, ultimately achieving a high image signal-to-noise ratio,” explained Bi Guoqiang.
Additionally, VISoR imaging technology offers compatibility and universality with various histological labeling techniques, solving the previous bottleneck problem where whole-brain transparency made deep uniform staining and transparent processing difficult.
Moreover, VISoR imaging technology demonstrates excellent scalability. As Bi Guoqiang explained, “In fact, larger samples result in higher imaging efficiency, so this technology can naturally scale from mouse brains to monkey brains and potentially apply to human brains in the future.”
Achieving high-precision, high-throughput analysis of various model animal brains may accelerate more accurate medical diagnosis and drug development

The applications of VISoR imaging technology are not limited to brain studies—it holds broad application potential in life sciences, medicine, and any field requiring large-scale 3D imaging.
For example, current clinical pathological diagnosis relies on two-dimensional microscopic images of tissue sections. Although artificial intelligence-based analysis methods are beginning to be applied to analyze and assist in diagnosing these images, the data itself has limitations—for instance, lacking full 3D structures, being heavily influenced by section angle and position, and involving relatively small data volumes.
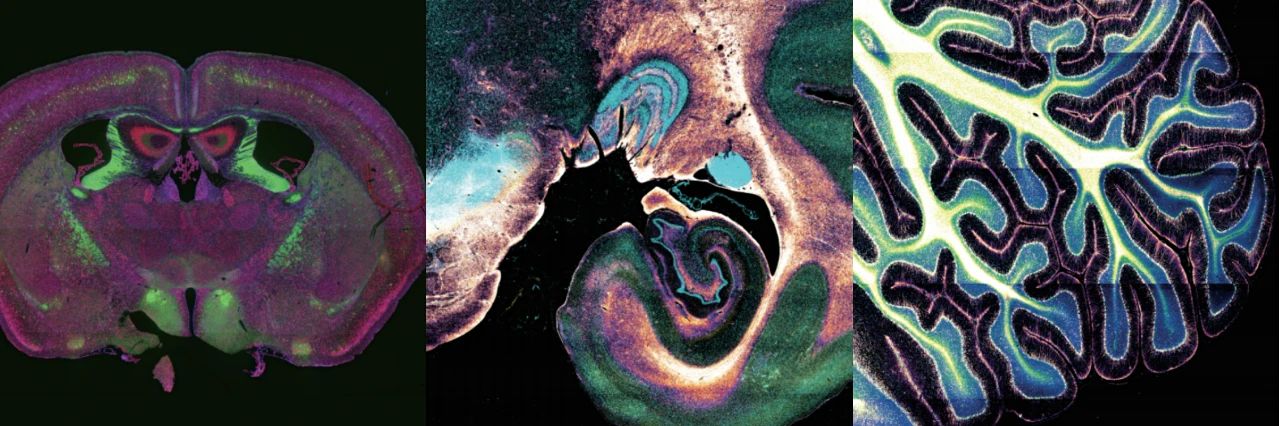
Image | Tissue structure images of different organs captured using VISoR technology (from left to right: mouse brain cross-section, macaque brain cross-section, macaque cerebellum) (Source: Interviewee)
Applying the high-speed VISoR imaging technology upgrades current 2D detection to high-throughput 3D imaging, better suited for AI big-data analysis. This fully utilizes biological, pathological, and surgical tissue samples, greatly enhancing the extraction of physiological and pathological information contained within them.
With the development of 3D imaging technologies like VISoR, research and pathological diagnosis based on complete 3D images of biological tissues are becoming a new scientific field.
Guoqiang Bi gave an example to DeepTech: “VISoR imaging is also highly applicable to developmental biology studies—for example, rapidly obtaining complete 3D structural information of embryos to understand their developmental processes and cellular molecular mechanisms.”

Image | Guoqiang Bi (Source: Interviewee)
At larger scales, anatomical studies over the past millennia have largely been conducted at the naked-eye level of resolution, with microscopic imaging applications limited to very small local areas.
With high-throughput microscopic imaging technologies like VISoR, it becomes feasible to perform cell or subcellular resolution imaging of entire organs or even the entire human body within a reasonable timeframe. “This could revolutionize anatomy, offering unprecedented clarity and digital descriptions of the complete architecture and structural details of the entire human body,” said Guoqiang Bi.
Currently, the team is expanding collaborations with hospitals, CRO companies, and investors who share a forward-looking vision. They are also partnering with AI-related enterprises to mine information from massive image datasets and organizing AI image analysis competitions to encourage broader community participation.
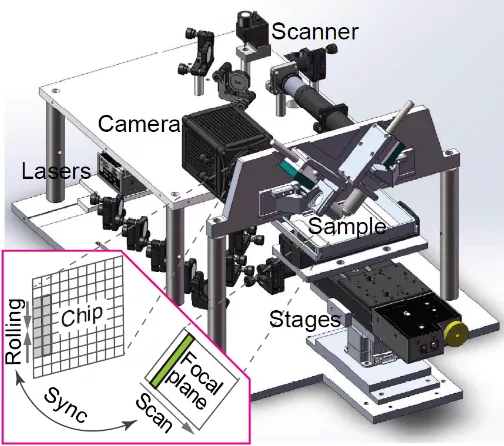
Image | VISoR engineering schematic (Source: Nature Biotechnology)
In summary, the combination of biomedical image explosions with artificial intelligence promises deeper insights into the fine structures of the human brain and bodily organs, enabling more precise understanding of disease-related changes, thereby accelerating medical diagnostics and pharmaceutical development.
Collaboration among multiple teams, interdisciplinary and diverse cooperation, with the ultimate goal of fully decoding the human brain

Since returning to China in 2007, Professor Guoqiang Bi has dedicated himself to imaging technology R&D and applications. Together with Professor Beiming Liu, he established interdisciplinary teams at USTC and the Shenzhen Institutes of Advanced Technology, focusing both on biological research addressing domain-specific challenges and on developing technologies grounded in physics principles, emphasizing problem-solving from first principles.
From concept to implementation of monkey brain imaging, the VISoR technology for brain atlas mapping took five years. Unlike traditional biological experiments, brain atlas research demands expertise from various disciplines.
The SMART workflow for brain atlas mapping includes sample preparation, processing, optical imaging, image processing, and data analysis—each step requiring specialized knowledge and varying researcher skill sets.
Image | Fang Xu with the VISoR high-speed 3D imaging equipment (Source: Interviewee)
"It is through long-term growth and development that we have built an interdisciplinary and diversified team. The team members include experts in optics, programming, neuroscience, etc. They have different professional backgrounds, expertise, and personality traits, all of which have been perfectly utilized in the project," said Xu Fang.
Currently, human understanding of primate brains still has significant room for exploration. This technology can directly expand humanity's knowledge of primate intelligence and brain-related diseases.
Xu Fang noted that the human brain is actually only about ten times larger than a monkey's brain, and the team's ultimate goal is to achieve complete mapping and understanding of the human brain.

Figure: First Authors of the Paper, from left to right: Xu Fang, Shen Yan, Ding Lufeng, Yang Zhaoyu (Source: Interviewee)
Bi Guoqiang believes that how to efficiently analyze and understand the related data will be the next challenge.
Based on existing technologies, laboratories around the world have conducted extensive whole-brain atlas imaging work on mice and generated massive datasets. However, it remains difficult to uncover patterns of neural network architecture or understand how a mouse processes information. Therefore, new computational methods and software tools need to be developed for deeper data mining.
In addition, at larger scales such as macaque and human brains, obtaining comprehensive brain atlas data remains a formidable task that requires more time, people, and resources to participate. "With the nation's increasing emphasis on brain science and continuous technological advancements, I believe there will be groundbreaking achievements in macaque and human brain atlas mapping within the next decade or two." said Bi Guoqiang.



